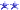DIY Zinc Plating - easier than I thought
#1
Melting Slicks


Thread Starter
Disclaimer: I have never done this before so there could be all sorts of ramifications in you doing this yourself. The chemicals used in Zinc plating can be very hazardous...proceed at your own risk!
I will up date this info as I see fit so it may change.
Note:
1. This recipe is enough to do hood latches.
2. ONLY PLATE ONE PART AT TIME!!
3. Always wear gloves and mask!!
Never touch the solution or breath the fumes!!
4. DO THIS OUTDOORS!!! The fumes are dangerous!!!
5. All parts have to be very clean and free of rust....sanded as necessary (the more polished before plating, the more shiny it will be). I used sprayed the parts with carb cleaner and let dry before plating.
When plating...the part must never touch the zinc!! Also, the Zinc will be attracted to the part and follow the path of least resistance while in the solution. You may get a "shadow" effect if you do not align your part to the Zinc part properly. It is a good idea to reorientate it periodically. Think of the zinc targeting the part like light (line of sight).
After reading a bunch of articles online on how to do Zinc plating, I decided to try it myself and found it a lot easier than I thought.
Anyone thinking of doing this will find that there are tons of recipes on what to use as the electrolyte and trying to figure out which one to go with can be confusing.
What I did was take everything I read and created my own recipe and hoped for the best...I was shocked at the results I got. Perhaps I lucked out but it was a lot easier and super cheap to do.
Allow me to explain what you will need...
GLOVES - EYE PROTECTION - DUST MASK IS A MUST!!
1. A plastic container to hold your part
2. A zinc source - I used two D-size HEAVY DUTY ZINC Batteries (cheap at dollar store)
3. Basic tools - plyers, screw driver, box cutter, tin snips/cutters
4. DC adapter (common cell phone, computer, radio, etc). I used a 6 volt/150ma DC adapter
5. Alligator clips/wire
6. 1 Gallon Distilled purified water
7. Epson Salt
8. White Vinegar 5% acidity
9. Sugar
10. Steel wool (000 grade)
11. Stainless steel wire for hanging parts
12. A piece of copper tubing to go on top of the container and hang your parts.
13. Either an aquarium air pump, air compressor or some kind of means to lightly agitate the water.
Steps...
Getting the zinc:
Watch some Youtube videos on disassembling batteries is helpful.
Wear gloves, mask and eye protection...the manganese chemicals in batteries is dangerous to your lungs! AND trust me when I say this...the manganese can get all over and VERY MESSY. If you get it on your carpet or your hands, you won't be able to get it out! It can get all over if you are not careful. Do this outside! You need a container to dump the manganese into.
1. Get 2 NEW FRESH ZINC batteries from Dollar store. Most Zinc batteries will say "HEAVY DUTY". DO NOT USE LITHIUM!!
2. Remove the plastic warp
3. Peal back the top and remove it by grabbing it with plyers.
4. Remove the center carbon rod using plyers.
5. Using a screw driver, remove the manganese inside over a container
6. The battery casing is the zinc you will need.
7. Using tin snips slice the zinc like a hotdog bun so it folds out.
8. Using steel wool clean the zinc - it is best to clean it right away as when the manganese dries, it gets like epoxy
9. Drill a small hole near an edge for a wire to hang the Zinc
10. Put this aside for now
Making the electrolyte
As I mentioned there are so many recipes out there...I decided to just jump into it and create my own. It's very simple.
The Epson salt allows the electricity flow. The sugar inhibits the zinc from creating large crystals. The vinegar is used as an acid/etching. The water is used to balance the PH.
For the size container you see...this is what I used.
1. 6 cups of White Vinegar (5% acidity)
2. 6 cups of distilled/purified water
3. 6 tablespoons of Epson salt
4. 6 tablespoons of sugar
Mix all the ingredients in the plastic container and stir until all of it is dissolved. Hang your Zinc plates in the solution at opposite ends of the container using stainless wire. I used stainless so that the chemicals do not pollute the electrolyte. Electrically connect the two zinc plates using insulated wire...keep this wire out of the solution.
Take your copper tubing and lay it on top of the container - it cannot touch the water. Take your part with the stainless wire and hang them on this tubing.
Connect up your DC power source....This is VERY IMPORTANT...the NEGATIVE needs to go to the part, the positive goes to the Zinc plates!! Just clip the negative on the copper tubing you put on top of the container. it should produce very very small bubbles...looking almost like smoke. If you have this, you're on the right path. You do not want large bubbles.
I let my parts soak for an hour. When you remove it, it should look a dull gray. Using 000-grade steel wool, clean the gray off...it should look shiny. After I cleaned the parts...washing with soap and rinsing, I put them back in for another hour. I did this 3 times. The more you do this the thicker the plating.
From what I read online...current is the key to it all. The equation is about 1ma/sq in of material you are plating. I just used the 6vm 150ma DC adapter. The less current you use, the longer the soaking. I read that most use 3 DC Volts by using 2 D-size batteries in series. I just found it a lot easier to use the adapter and not worry about the batteries depleting.
Good luck!!
I will up date this info as I see fit so it may change.
Note:
1. This recipe is enough to do hood latches.
2. ONLY PLATE ONE PART AT TIME!!
3. Always wear gloves and mask!!
Never touch the solution or breath the fumes!!
4. DO THIS OUTDOORS!!! The fumes are dangerous!!!
5. All parts have to be very clean and free of rust....sanded as necessary (the more polished before plating, the more shiny it will be). I used sprayed the parts with carb cleaner and let dry before plating.
When plating...the part must never touch the zinc!! Also, the Zinc will be attracted to the part and follow the path of least resistance while in the solution. You may get a "shadow" effect if you do not align your part to the Zinc part properly. It is a good idea to reorientate it periodically. Think of the zinc targeting the part like light (line of sight).
After reading a bunch of articles online on how to do Zinc plating, I decided to try it myself and found it a lot easier than I thought.
Anyone thinking of doing this will find that there are tons of recipes on what to use as the electrolyte and trying to figure out which one to go with can be confusing.
What I did was take everything I read and created my own recipe and hoped for the best...I was shocked at the results I got. Perhaps I lucked out but it was a lot easier and super cheap to do.
Allow me to explain what you will need...
GLOVES - EYE PROTECTION - DUST MASK IS A MUST!!
1. A plastic container to hold your part
2. A zinc source - I used two D-size HEAVY DUTY ZINC Batteries (cheap at dollar store)
3. Basic tools - plyers, screw driver, box cutter, tin snips/cutters
4. DC adapter (common cell phone, computer, radio, etc). I used a 6 volt/150ma DC adapter
5. Alligator clips/wire
6. 1 Gallon Distilled purified water
7. Epson Salt
8. White Vinegar 5% acidity
9. Sugar
10. Steel wool (000 grade)
11. Stainless steel wire for hanging parts
12. A piece of copper tubing to go on top of the container and hang your parts.
13. Either an aquarium air pump, air compressor or some kind of means to lightly agitate the water.
Steps...
Getting the zinc:
Watch some Youtube videos on disassembling batteries is helpful.
Wear gloves, mask and eye protection...the manganese chemicals in batteries is dangerous to your lungs! AND trust me when I say this...the manganese can get all over and VERY MESSY. If you get it on your carpet or your hands, you won't be able to get it out! It can get all over if you are not careful. Do this outside! You need a container to dump the manganese into.
1. Get 2 NEW FRESH ZINC batteries from Dollar store. Most Zinc batteries will say "HEAVY DUTY". DO NOT USE LITHIUM!!
2. Remove the plastic warp
3. Peal back the top and remove it by grabbing it with plyers.
4. Remove the center carbon rod using plyers.
5. Using a screw driver, remove the manganese inside over a container
6. The battery casing is the zinc you will need.
7. Using tin snips slice the zinc like a hotdog bun so it folds out.
8. Using steel wool clean the zinc - it is best to clean it right away as when the manganese dries, it gets like epoxy
9. Drill a small hole near an edge for a wire to hang the Zinc
10. Put this aside for now
Making the electrolyte
As I mentioned there are so many recipes out there...I decided to just jump into it and create my own. It's very simple.
The Epson salt allows the electricity flow. The sugar inhibits the zinc from creating large crystals. The vinegar is used as an acid/etching. The water is used to balance the PH.
For the size container you see...this is what I used.
1. 6 cups of White Vinegar (5% acidity)
2. 6 cups of distilled/purified water
3. 6 tablespoons of Epson salt
4. 6 tablespoons of sugar
Mix all the ingredients in the plastic container and stir until all of it is dissolved. Hang your Zinc plates in the solution at opposite ends of the container using stainless wire. I used stainless so that the chemicals do not pollute the electrolyte. Electrically connect the two zinc plates using insulated wire...keep this wire out of the solution.
Take your copper tubing and lay it on top of the container - it cannot touch the water. Take your part with the stainless wire and hang them on this tubing.
Connect up your DC power source....This is VERY IMPORTANT...the NEGATIVE needs to go to the part, the positive goes to the Zinc plates!! Just clip the negative on the copper tubing you put on top of the container. it should produce very very small bubbles...looking almost like smoke. If you have this, you're on the right path. You do not want large bubbles.
I let my parts soak for an hour. When you remove it, it should look a dull gray. Using 000-grade steel wool, clean the gray off...it should look shiny. After I cleaned the parts...washing with soap and rinsing, I put them back in for another hour. I did this 3 times. The more you do this the thicker the plating.
From what I read online...current is the key to it all. The equation is about 1ma/sq in of material you are plating. I just used the 6vm 150ma DC adapter. The less current you use, the longer the soaking. I read that most use 3 DC Volts by using 2 D-size batteries in series. I just found it a lot easier to use the adapter and not worry about the batteries depleting.
Good luck!!
Last edited by jusplainwacky; 11-19-2015 at 03:39 PM.
The following 5 users liked this post by jusplainwacky:
65air_coupe (11-26-2022),
ifitgoesfast (11-19-2015),
interpon (10-04-2019),
Mako72 (11-20-2015),
Pop Chevy (11-19-2015)
#2
Melting Slicks


Thread Starter
There is a video file at the end of my post above...the large bubbles you see in the back ground is from the air compressor...not the plating process. You can see the small bubbles developing.
Last edited by jusplainwacky; 11-19-2015 at 01:34 PM.
#4
Le Mans Master


Live well,
SJW
#5
Melting Slicks


Thread Starter
Mike...again, I'm now pro here, but my understanding that the solution can be used over and over...in fact the more you plate, the more the solution is saturated with the Zinc making the plating go faster. So I plan to put it into a container and store it for next time. I would never dump/pollute our environment. I actually own a company that developed a device for the detection of toxic lead to show you were I stand on this subject.
SJW....I cannot speak to this cause I am not a chemist, but I do know the dust from the magnesium inside the battery is dangerous. I just had to warm people to be extra cautious. I did all this with a very crude setup...just to test the process and started off with a bolt. That worked so good, that I just decided to not move/change a thing.
I read that you can get Zinc roofing strips from Home Depot that keeps moss off roofs...and you can also get it from a boating store as they use a sacrificing zinc anode on boats.
I just decided the local Dollar Store was easier and cheaper. It maybe why my parts came out so shiny....was the zinc they use...it maybe a Zinc/tin combo.
SJW....I cannot speak to this cause I am not a chemist, but I do know the dust from the magnesium inside the battery is dangerous. I just had to warm people to be extra cautious. I did all this with a very crude setup...just to test the process and started off with a bolt. That worked so good, that I just decided to not move/change a thing.
I read that you can get Zinc roofing strips from Home Depot that keeps moss off roofs...and you can also get it from a boating store as they use a sacrificing zinc anode on boats.
I just decided the local Dollar Store was easier and cheaper. It maybe why my parts came out so shiny....was the zinc they use...it maybe a Zinc/tin combo.
Last edited by jusplainwacky; 11-19-2015 at 02:09 PM.
The following users liked this post:
Wjkiefiuk@comcast.ne (07-03-2023)
#6
Melting Slicks


Thread Starter
Here is a picture showing you what the part looks like when you take it out of the bath. As I mentioned, it's very dull gray looking. I used steel wool to remove the dullness on half of it to show the difference. It's hard to see it with the camera and shadowing. The parts are very shiny.
The following users liked this post:
raylag64 (11-19-2015)
#7
Melting Slicks


Thread Starter
Finished up my hood brackets and accelerator rod. The rod came out looking really shiny...the picture doesn't do it justice.
Last edited by jusplainwacky; 11-19-2015 at 08:46 PM.
#8
Drifting


#9
Melting Slicks


Thread Starter
This is true, but it comes with a price and then you get to wait. I'm impatient.
I decided to do it myself for a couple of reasons...
One was just for the education and to see if I could, and
Two is that I now can plate anything I want and not have to run to the plating shop...especially for small parts.
I decided to do it myself for a couple of reasons...
One was just for the education and to see if I could, and
Two is that I now can plate anything I want and not have to run to the plating shop...especially for small parts.
The following users liked this post:
65air_coupe (11-26-2022)
#11
Le Mans Master



The following users liked this post:
65air_coupe (11-26-2022)
#12
Melting Slicks


Thread Starter
I'm sure there are many places you can get Zinc including a marine supply and Home Depot I mentioned, but you have to typically buy a heck of a lot more than you will ever need...as compared to a buck for a few batteries at the dollar store. Call me cheap.
I just question if the Zinc used in the battery is a Zinc alloy of some type...maybe Zinc/Tin or Aluminum because my parts came out real shiny. Like I said, I'm no pro at this, but if I had to do it again, I'd stick to my recipe cause I got such amazing results.
I just question if the Zinc used in the battery is a Zinc alloy of some type...maybe Zinc/Tin or Aluminum because my parts came out real shiny. Like I said, I'm no pro at this, but if I had to do it again, I'd stick to my recipe cause I got such amazing results.
#13
Le Mans Master


I'm sure there are many places you can get Zinc including a marine supply and Home Depot I mentioned, but you have to typically buy a heck of a lot more than you will ever need...as compared to a buck for a few batteries at the dollar store. Call me cheap.
I just question if the Zinc used in the battery is a Zinc alloy of some type...maybe Zinc/Tin or Aluminum because my parts came out real shiny. Like I said, I'm no pro at this, but if I had to do it again, I'd stick to my recipe cause I got such amazing results.
I just question if the Zinc used in the battery is a Zinc alloy of some type...maybe Zinc/Tin or Aluminum because my parts came out real shiny. Like I said, I'm no pro at this, but if I had to do it again, I'd stick to my recipe cause I got such amazing results.
If you do, please let us know how it turns out!
Live well,
SJW
Last edited by SJW; 11-19-2015 at 10:27 PM.
#14
Le Mans Master


I'm sure there are many places you can get Zinc including a marine supply and Home Depot I mentioned, but you have to typically buy a heck of a lot more than you will ever need...as compared to a buck for a few batteries at the dollar store. Call me cheap.
I just question if the Zinc used in the battery is a Zinc alloy of some type...maybe Zinc/Tin or Aluminum because my parts came out real shiny. Like I said, I'm no pro at this, but if I had to do it again, I'd stick to my recipe cause I got such amazing results.
I just question if the Zinc used in the battery is a Zinc alloy of some type...maybe Zinc/Tin or Aluminum because my parts came out real shiny. Like I said, I'm no pro at this, but if I had to do it again, I'd stick to my recipe cause I got such amazing results.

The following users liked this post:
65air_coupe (11-26-2022)
#15
Melting Slicks


Thread Starter
Steve...I have no intention of trying it with a pure Zinc source...but it would be interesting to see if the results are different.
I have a Zinc source on my boat and it's gray looking...but I'm confident that's just cause it's oxidized.
Makes me wonder if this is the reason that some people say their parts didn't come out very shiny and more dark looking.
Might have to do some research to find out what kind of Zinc (or alloy) is used in making the batteries.
I have a Zinc source on my boat and it's gray looking...but I'm confident that's just cause it's oxidized.
Makes me wonder if this is the reason that some people say their parts didn't come out very shiny and more dark looking.
Might have to do some research to find out what kind of Zinc (or alloy) is used in making the batteries.
#16
Melting Slicks


Thread Starter
I did some research online and found out that the Zinc used in a battery is considered ultra-pure at 99.9%. Interesting.
The following users liked this post:
Lee05 (11-24-2015)
#17
Race Director


#20
Melting Slicks


I've never used it but, an inexpensive way to get shiny parts right out of the bath may be:
http://www.caswellplating.com/copy-c...ener-4-oz.html
I used Caswell's black oxide on a lit of my original bolts, it works well for what it is.
http://www.caswellplating.com/copy-c...ener-4-oz.html
I used Caswell's black oxide on a lit of my original bolts, it works well for what it is.